505(b)(1)
LXP108
LXP108 is a new chemical entity, and in our plan, it will treat carcinoma related to the digestive system.
適應症
實體腫瘤
- 腎臟腫瘤
- 肝癌
- 胰臟癌
Science
多靶點激酶抑制劑 (Multi-target Kinase Inhibitor)
LXP108 為多靶點激酶抑制劑,可有效抑制多條癌細胞增生的訊號路徑
優勢:
- 更有效的抑制癌細胞生長
- 降低副作用
- 克服抗藥性
臨床上也證明多靶點藥物比單一靶點藥物可以更有效的抑制癌腫瘤細胞的生長,展現更好更廣的抗癌效果
最新進展
- 於2024年進入第一階段臨床試驗
- 美國FDA及台灣TFDA IND申請中
臨床前
IND 申請
臨床一期
臨床二期
臨床三期
市場
Phase1
Pre-clinical 臨床前研究
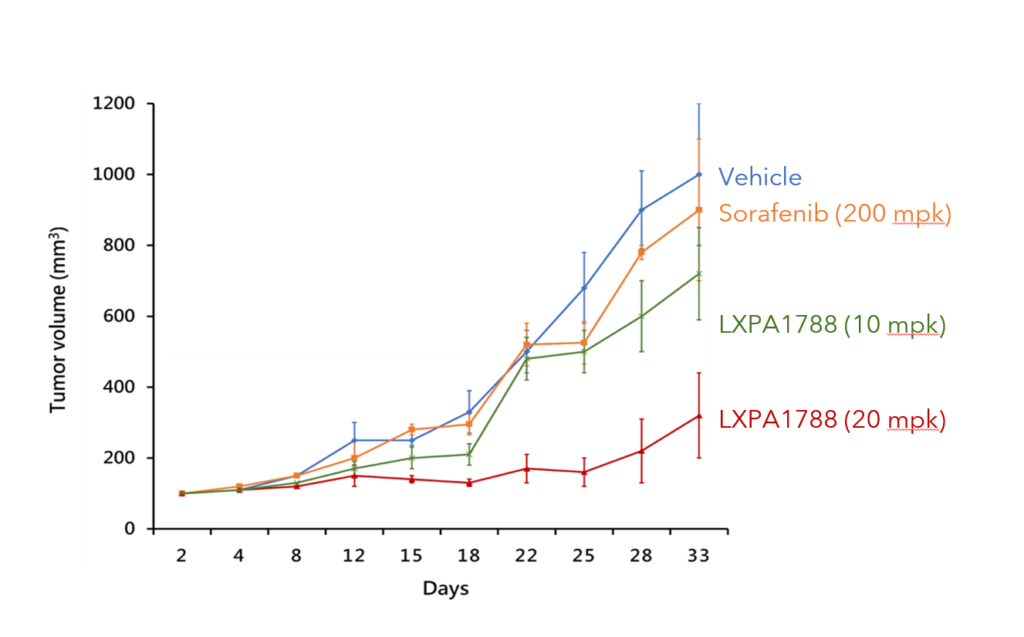
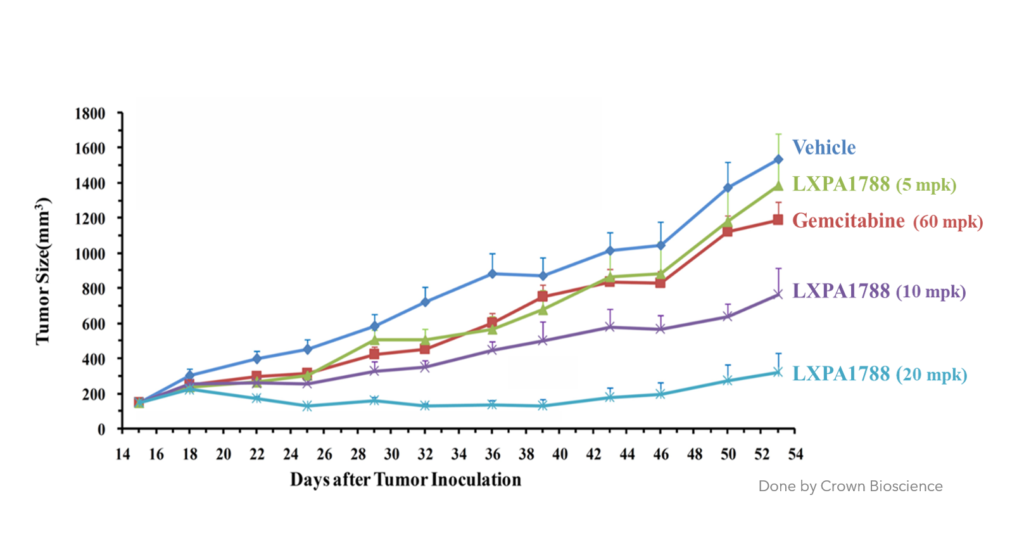
LXP108 對 胰臟癌及肝癌可有效抑制 (動物試驗)
胰臟癌
肝癌
比較藥物相關說明
Linifanib
- 抑制受體酪胺酸激酶(RTK)、血管內皮生長因子(VEGF)和血小板衍生生長因子(PDGF)
- 目前處於第三期臨床試驗。
- 適應症
肝細胞癌
Sorafenib
- 抑制Raf激酶、PDGF(血小板衍生生長因子)、VEGF受體2和3激酶,以及c-Kit(幹細胞因子受體)。
- 於2005年12月20日首次獲得FDA批准。
- 適應症
2005年:晚期腎細胞癌
2007年:無法切除的肝細胞癌
2013年:轉移性分化型甲狀腺癌(Metastatic Differentiated Thyroid Cancer)
Midostaurin
- 抑制蛋白激酶Cα、VEGFR2、KIT、PDGFR以及野生型/突變型FLT3酪胺酸激酶。
- 於2017年4月28日獲得FDA批准。
- 適應症
2017年:FLT3突變型急性骨髓性白血病(AML)及系統性肥大細胞增多症(Systemic Mastocytosis)
專利
取得美國、中華民國、南韓、中國、歐洲,總計 17個地區。
- United States
- Taiwan
- Korea
- China
- Europe
