505(b)(1)
LXP108
LXP108 is a new chemical entity, and in our plan, it will treat carcinoma related to the digestive system.
Indications
Solid Tumor
- Renal Cancer
- Liver Cancer
- Pancreatic Cancer
Science
Multi-target Kinase Inhibitor
LXP108 is a multi-target kinase inhibitor that simultaneously inhibits multiple signaling pathways essential for cancer cell proliferation.
Benefits of multi-target kinase inhibitor:
- More effectively inhibits cancer cell growth
- Reduces side effects
- Overcomes drug resistance
Lates Progress
- Enter Phase 1 Clinical Trial in 2024
- FDA and TFDA IND Application
Preclinical
IND
Phase 1
Phase 2
Phase 3
Market
Phase1
Pre-clinical Results
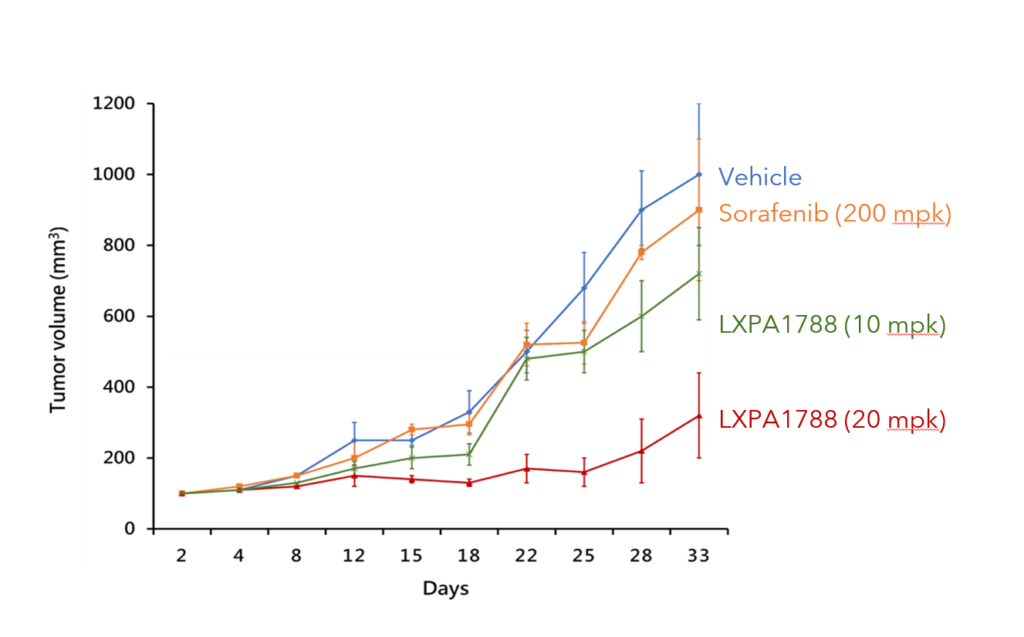
LXP108 can effectively inhibit pancreatic and liver cancer (animal studies)
Pancreatic Cancer
Liver Cancer
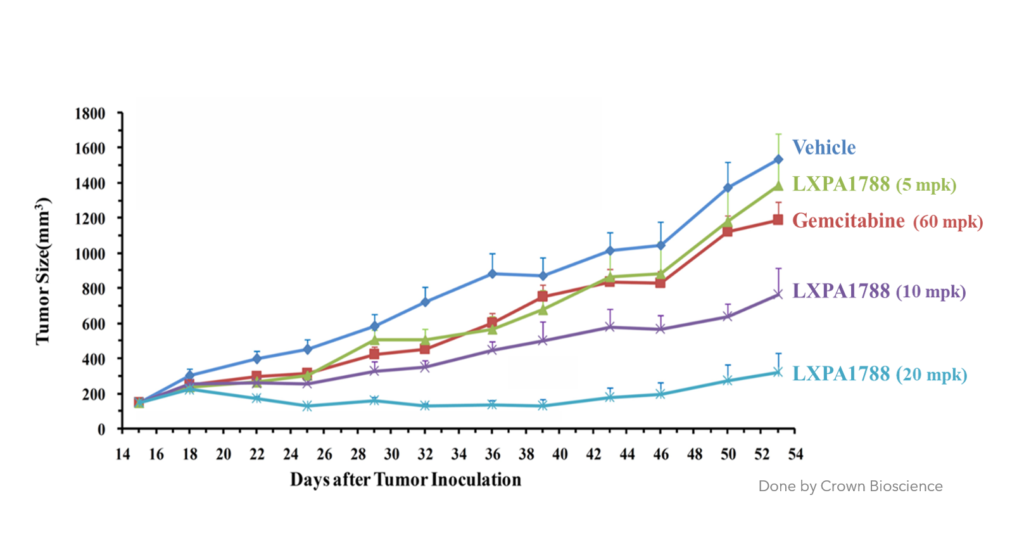
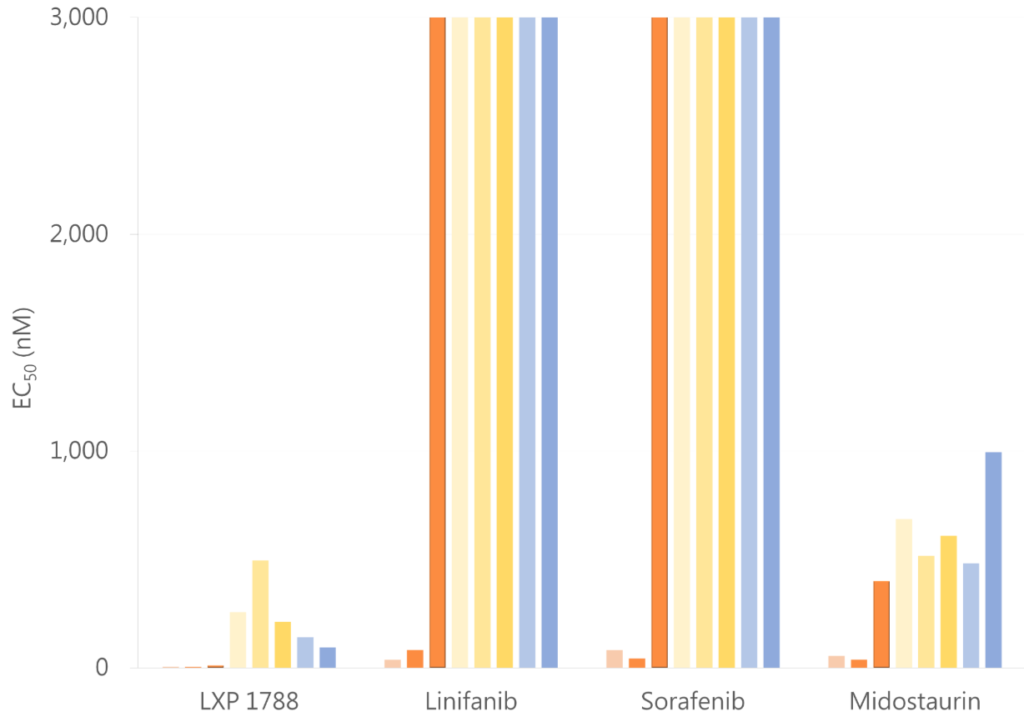
Compare to other drug
LXP108 has better inhibitory effect
Cell lines:
MOLM-13
Acute Monocytic Leukemia Cell Line
The cell line was established from the peripheral blood of a patient at relapse of acute monocytic leukemia which had evolved from myelodysplastic syndrome (MDS).
MV4-11
Acute Monocytic Leukemia Cell Line
The cell line was established from the peripheral blood of a patient at relapse of acute monocytic leukemia which had evolved from myelodysplastic syndrome (MDS).
RS4-11
Acute Monocytic Leukemia Cell Line
A lymphoblast cell line that was isolated from the marrow of a White, 32 years old, female patient with acute lymphoblastic leukemia.
HCC827
Non-Small Cell Lung Cancer Cell Line
An epithelial cell that was isolated from the lung of a White, 39-year-old female patient with adenocarcinoma.
H1975
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from the lungs of a nonsmoking female with non-small cell lung cancer.
H2228
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from a lung adenocarcinoma derived from a female nonsmoker with non-small cell lung cancer.
HCT-116
Colon Cancer Cell Line
The cell line was isolated from the colon of an adult male with colon cancer.
Mia-PaCa2
Pancreatic Cancer Cell Line
An epithelial cell line that was derived from tumor tissue of the pancreas obtained from a 65-year-old, White male.
Comparison of competitor
Linifanib
- It’s a inhibitor of receptor tyrosine kinase (RTK), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptor families.
- It’s still on phase III clinical trial.
- Indication(s):
Hepatocellular Carcinoma
Sorafenib
- It’s a inhibitor of Raf kinase, PDGF (platelet-derived growth factor), VEGF receptor 2 & 3 kinases and c Kit the receptor for Stem cell factor.
- First approved by FDA in December 20, 2005.
- Indication(s):
2005 Advanced Renal Cell Carcinoma
2007 Unresectable Hepatocellular Carcinoma
2013 Metastatic Differentiated Thyroid Cancer
Midostaurin
- It’s a inhibitor of protein kinase C alpha, VEGFR2, KIT, PDGFR and WT and/or mutant FLT3 tyrosine kinases.
- First approved by FDA in April 28, 2017.
- Indication(s):
2017 FLT3-Mutated Acute Myeloid Leukemia and Systemic Mastocytosis
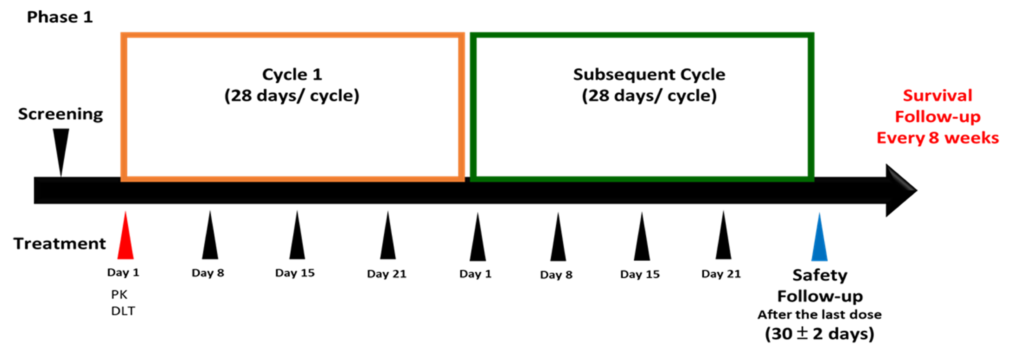
Clinical Study Design
Phase I clinical trial is planned to be conducted in 2024 Q2
3 Taiwan Hospitals
Patients with solid tumors
30-40 clinical trial participants
Adaptive 3+3
stimated once every six months
Estimated once weekly injection
Patents
Patents have been granted in Taiwan, the United States, Korea, China and Europe.
- United States
- Taiwan
- Korea
- China
- Europe

