505(b)(1)
LXPA1788
LXPA1788是全新藥物分子,目前規劃將用於消化道系統相關之癌症治療。
適應症
實體腫瘤
- 腎臟腫瘤
- 肝癌
- 胰臟癌
Science
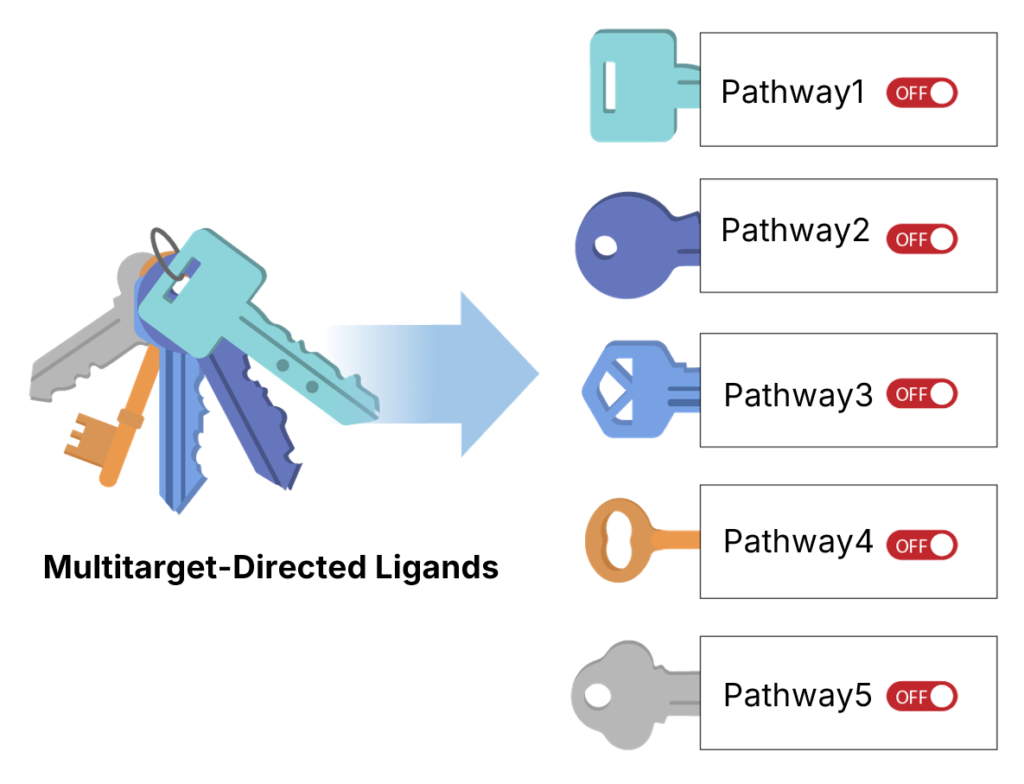
多靶點激酶抑制劑 (Multi-target Kinase Inhibitor)
LXPA1788 是一種多靶點激酶抑制劑,能同時抑制多條對癌細胞增生至關重要的訊息傳遞路徑
優勢:
- 更有效的抑制癌細胞生長
- 降低副作用
- 克服抗藥性
最新進展
- 於2024年進入第一階段臨床試驗
- 美國FDA及台灣TFDA IND申請中
臨床前(Preclinical)
申請(IND)
第一階段(Phase 1)
第二階段(Phase 2)
第三階段(Phase 3)
市場(Market)
Phase1
Pre-clinical 臨床前研究
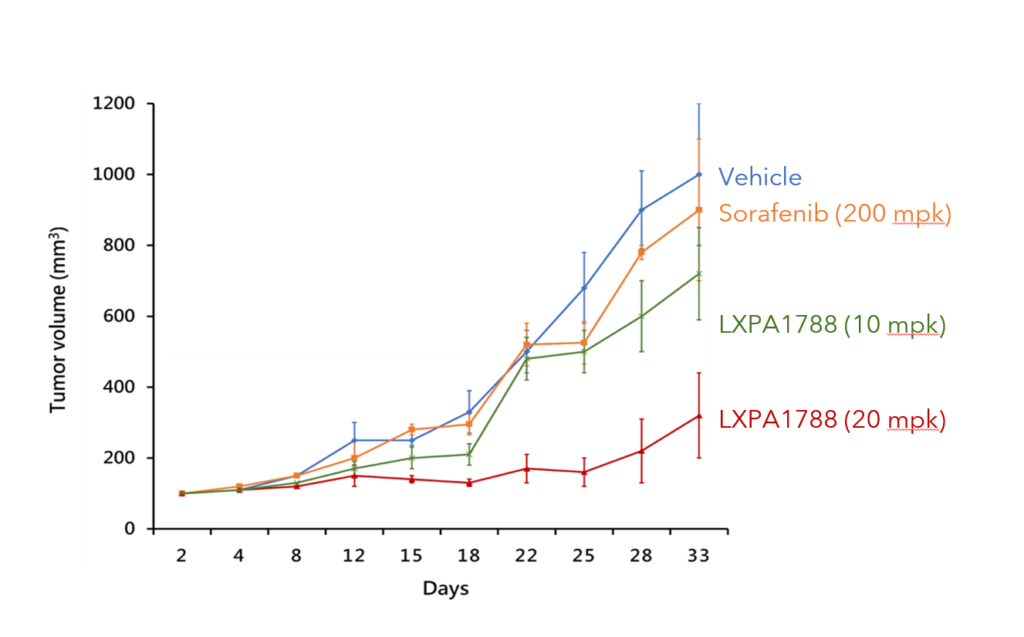
LXPA1788可有效抑制 胰臟癌及肝癌 (動物試驗)
胰臟癌
肝癌
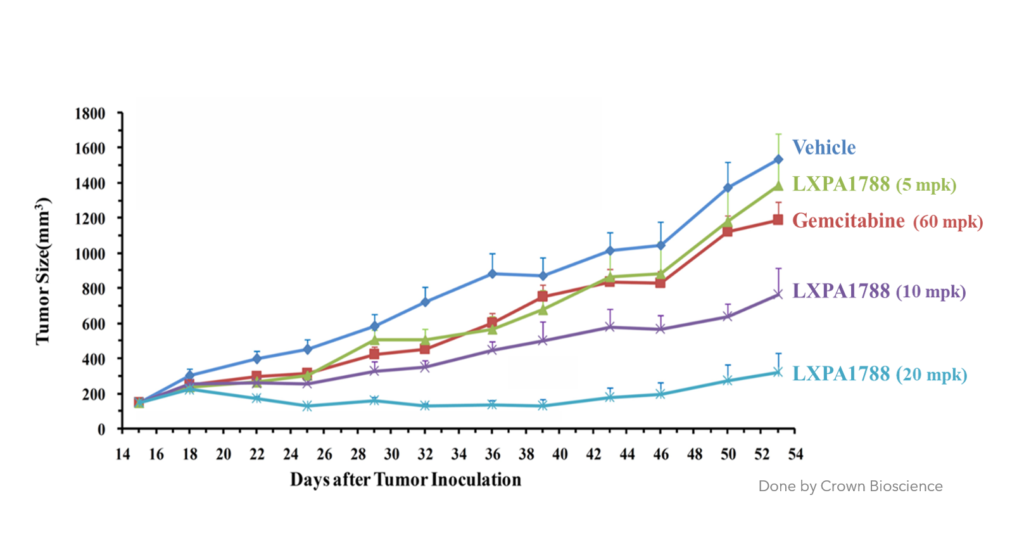
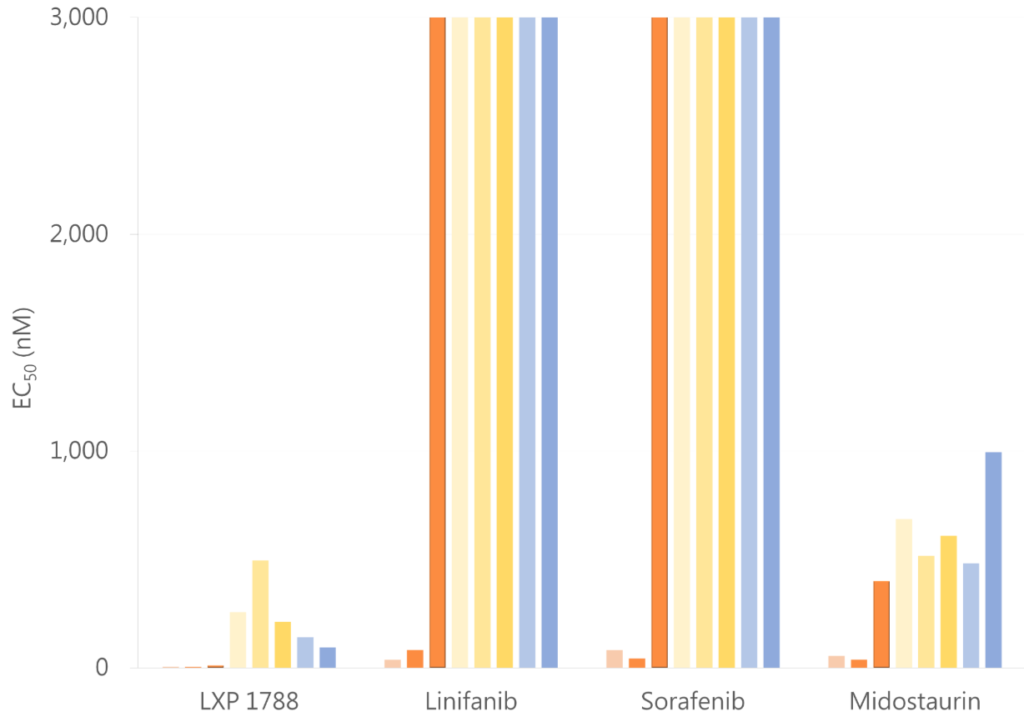
Compare to other drug 相對於其他藥物
LXPA1788具有 更好的抑制效果
癌症細胞株說明
MOLM-13
Acute Monocytic Leukemia Cell Line
The cell line was established from the peripheral blood of a patient at relapse of acute monocytic leukemia which had evolved from myelodysplastic syndrome (MDS).
MV4-11
Acute Monocytic Leukemia Cell Line
The cell line was established from the peripheral blood of a patient at relapse of acute monocytic leukemia which had evolved from myelodysplastic syndrome (MDS).
RS4-11
Acute Monocytic Leukemia Cell Line
A lymphoblast cell line that was isolated from the marrow of a White, 32 years old, female patient with acute lymphoblastic leukemia.
HCC827
Non-Small Cell Lung Cancer Cell Line
An epithelial cell that was isolated from the lung of a White, 39-year-old female patient with adenocarcinoma.
H1975
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from the lungs of a nonsmoking female with non-small cell lung cancer.
H2228
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from a lung adenocarcinoma derived from a female nonsmoker with non-small cell lung cancer.
HCT-116
Colon Cancer Cell Line
The cell line was isolated from the colon of an adult male with colon cancer.
Mia-PaCa2
Pancreatic Cancer Cell Line
An epithelial cell line that was derived from tumor tissue of the pancreas obtained from a 65-year-old, White male.
比較藥物相關說明
Linifanib
- 抑制受體酪胺酸激酶(RTK)、血管內皮生長因子(VEGF)和血小板衍生生長因子(PDGF)
- 目前處於第三期臨床試驗。
- 適應症
肝細胞癌
Sorafenib
- 抑制Raf激酶、PDGF(血小板衍生生長因子)、VEGF受體2和3激酶,以及c-Kit(幹細胞因子受體)。
- 於2005年12月20日首次獲得FDA批准。
- 適應症
2005年:晚期腎細胞癌
2007年:無法切除的肝細胞癌
2013年:轉移性分化型甲狀腺癌(Metastatic Differentiated Thyroid Cancer)
Midostaurin
- 抑制蛋白激酶Cα、VEGFR2、KIT、PDGFR以及野生型/突變型FLT3酪胺酸激酶。
- 於2017年4月28日獲得FDA批准。
- 適應症
2017年:FLT3突變型急性骨髓性白血病(AML)及系統性肥大細胞增多症(Systemic Mastocytosis)
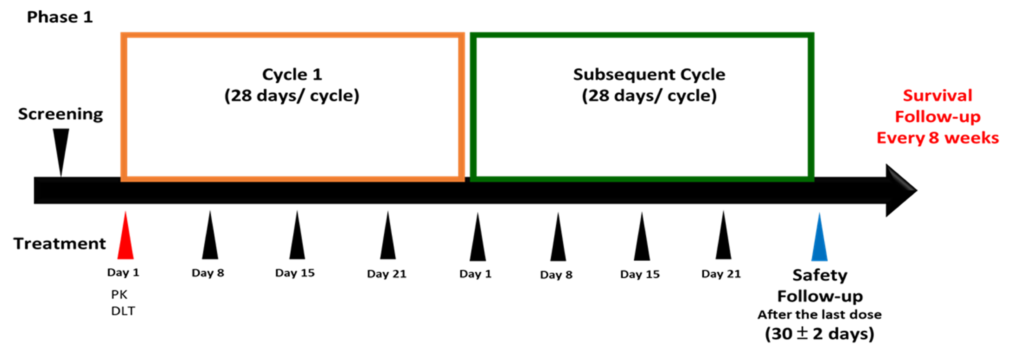
Clinical Study Design 臨床試驗之規劃
Phase I 臨床試驗預計於 2024 Q2進行
台灣:三家執行醫院
實體腫瘤病患
預計收30~40名臨床受試者
Adaptive 3+3
預計一次療程為六個月
預計一周注射藥物一次
專利
取得美國、中華民國、南韓、中國、歐洲,總計 17個地區。
- United States
- Taiwan
- Korea
- China
- Europe

