505(b)(2)
LXPB5268
LXPB5268 was initially designed as a psychiatric medication, but it has been further developed for cancer treatment.
Indications
Triple-negative breast cancer
Current Treatment Methods:
Chemotherapy ➡️ Surgery
Challenges with Current Treatment Methods:
- Triple-negative breast cancer represents approximately 15-20% of breast cancer cases, with only 15% eligible for targeted therapies.
- The objective response rate does not reach 100% (typically 60-90%).
- Tumor shrinkage rate is relatively low (generally around 40-50%).
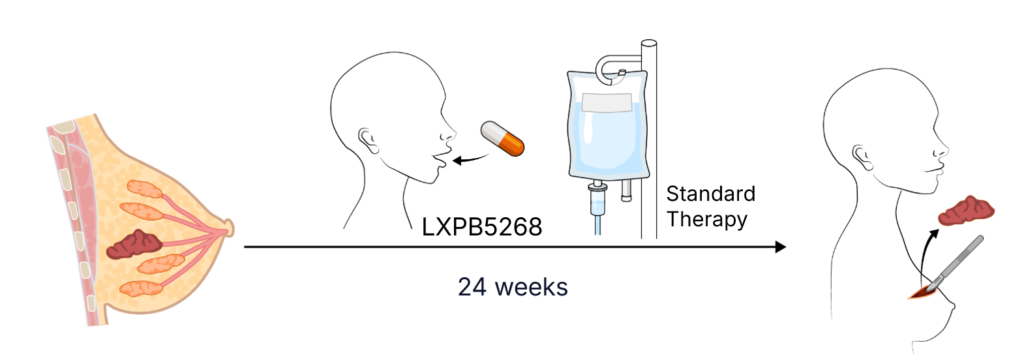
New Treatment NACT + LXPB5268
Standard Neoadjuvant Chemotherapy (NACT) combines LXPB5268
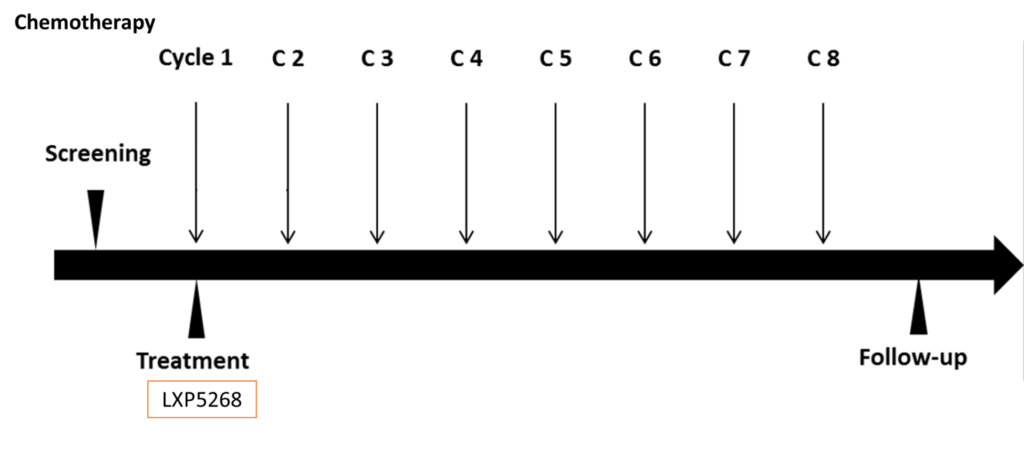
Clinical Trial
The proof-of-concept clinical trial for breast cancer has been conducted at a medical center in central Taiwan.
Expected Enrollment
18 people
Estimated completion of enrollment
September 2024
First participant enrollment date
February 2022
First participant completion date
August 2022
Latest Progress
Preclinical
IND
Phase 1
Phase 2
Phase 3
Market
Phase 2 (Proof of Concept)
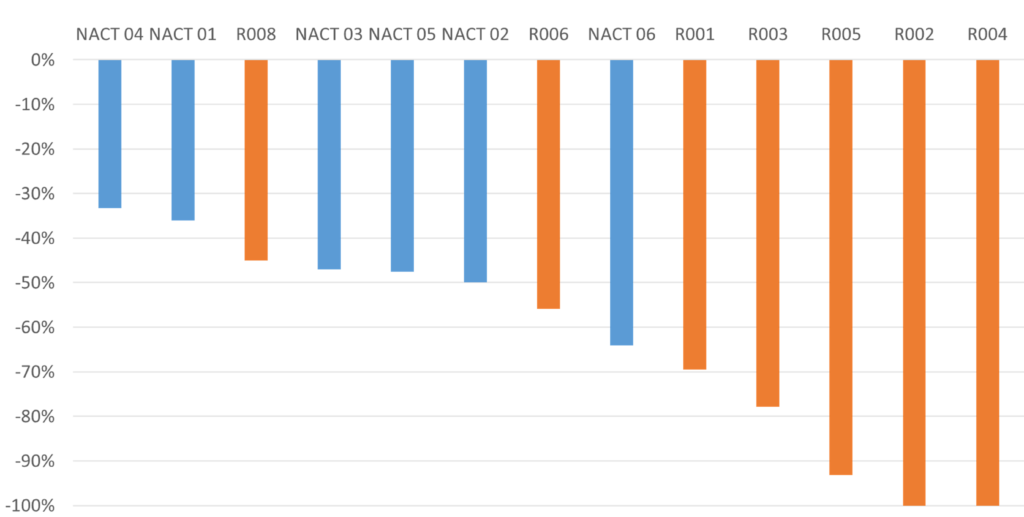
Results
Better tumor reduction, Higher rate of pathological complete response
The LXPB5268+NACT group showed better tumor reduction, and a higher rate of pathological complete response compared to the NACT group alone.
Significant reduction observed in CT images
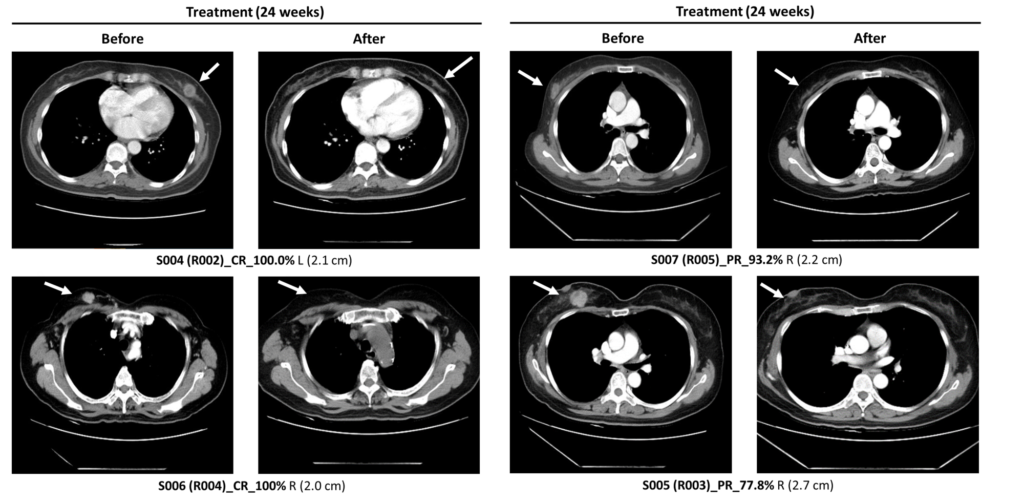
LXPB5268 combination therapy with NACT better than NACT only
Patents
Patents for cancer-related indications have been granted in Taiwan, the United States, Japan, and Australia, with the European patent currently pending.
- Taiwan
- United States
- Japan
- Australia
- Europe

