505(b)(1)
LXPA5948
LXPA5948 has obtained patents in seven countries. Animal studies suggest that LXPA5948, when combined with Osimertinib for non-small cell lung cancer, delays resistance to the treatment. Preclinical studies and new formulations are currently underway.
Indications
Non-Small Cell Lung Cancer & Alzheimer's disease
Development Value
Animal test results suggest that LXPA5948 is more effective when used in combination with Osimertinib, the current third-generation treatment for EGFR-mutated non-small cell lung cancer.
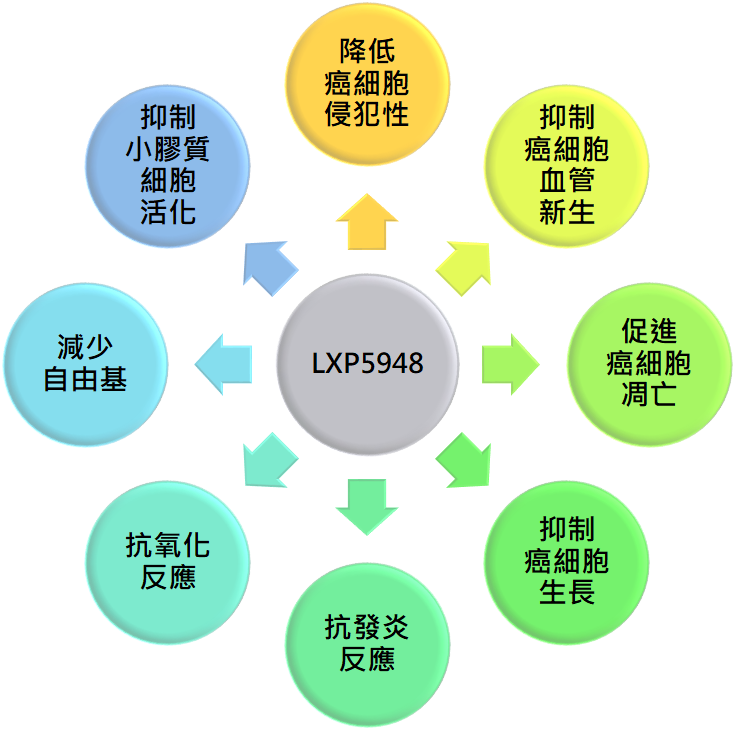
Clinical Trial
Latest Progress
Preclinical
IND
Phase 1
Phase 2
Phase 3
Market
Preclinical
Patents
Patents have been granted in Taiwan and the United States, with applications filed in other countries currently in progress.
- Taiwan
- United States
