505(b)(1)
LXP107
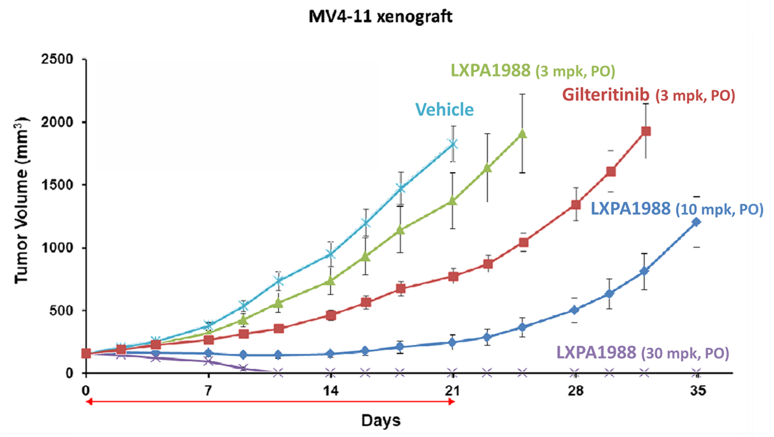
This product is a new chemical entity (NCE), specifically a kinase inhibitor targeting FLT3. Animal studies show that LXP107 also effectively inhibits mutated FLT3. Currently, preclinical studies and new formulation designs are underway.
Indications
FLT3-mutated Acute Myeloid Leukemia (AML)
Epidemiology
- Globally, approximately 190,000 new AML cases are reported annually.
- The number of AML patients with FLT3 mutations is around 65,000 (30-35%).
- It is classified as an orphan disease (affecting < 200,000 people)
Development strengths
- Accelerated review for market approval (authorized by EMA in December 2020 for aspacytarabine).
- Tax incentives for clinical expenses (US – 25% off).
- Post-approval market exclusivity for seven years (US) / ten years (Taiwan).
- As of 2016, the median annual cost of orphan drugs exceeded $32,000.
Lates Progress
Preclinical
IND
Phase 1
Phase 2
Phase 3
Market
Preclinical